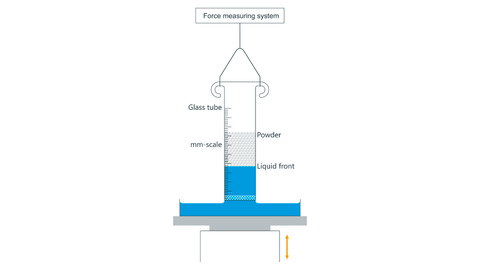
Washburn Method
The Washburn method measures the contact angle and surface free energy of porous materials like powders and textiles by observing how a liquid is drawn up into the material via capillary action.
A glass tube filled with powder is dipped in a test liquid, and the liquid’s mass uptake over time is recorded. Treating the powder as many tiny capillaries, the relationship between mass squared (m2m^2m2) and time (ttt) is linear and described by the Washburn equation:
m2=cσcosθηρ×tm^2 = \frac{c \sigma \cos \theta}{\eta \rho} \times tm2=ηρcσcosθ×t
- ccc is a capillary constant related to pore size and packing,
- θ\thetaθ is the contact angle,
- σ,η,ρ\sigma, \eta, \rhoσ,η,ρ are the liquid’s surface tension, viscosity, and density.
To find ccc, measure with a fully wetting liquid (contact angle 0∘0^\circ0∘), then use this to calculate θ\thetaθ for other liquids.
Notes:
- Powder packing must be consistent since ccc depends on bulk density.
- Angles above 90° cannot be measured (no wetting). For these, drop shape analysis is used instead.

 Surface Tension Instruments
Surface Tension Instruments  Foam Analyzers
Foam Analyzers  High Pressure Reactors and Pilot Plants
High Pressure Reactors and Pilot Plants  Material Testing Service
Material Testing Service  Emulsion & Suspension Analysis
Emulsion & Suspension Analysis