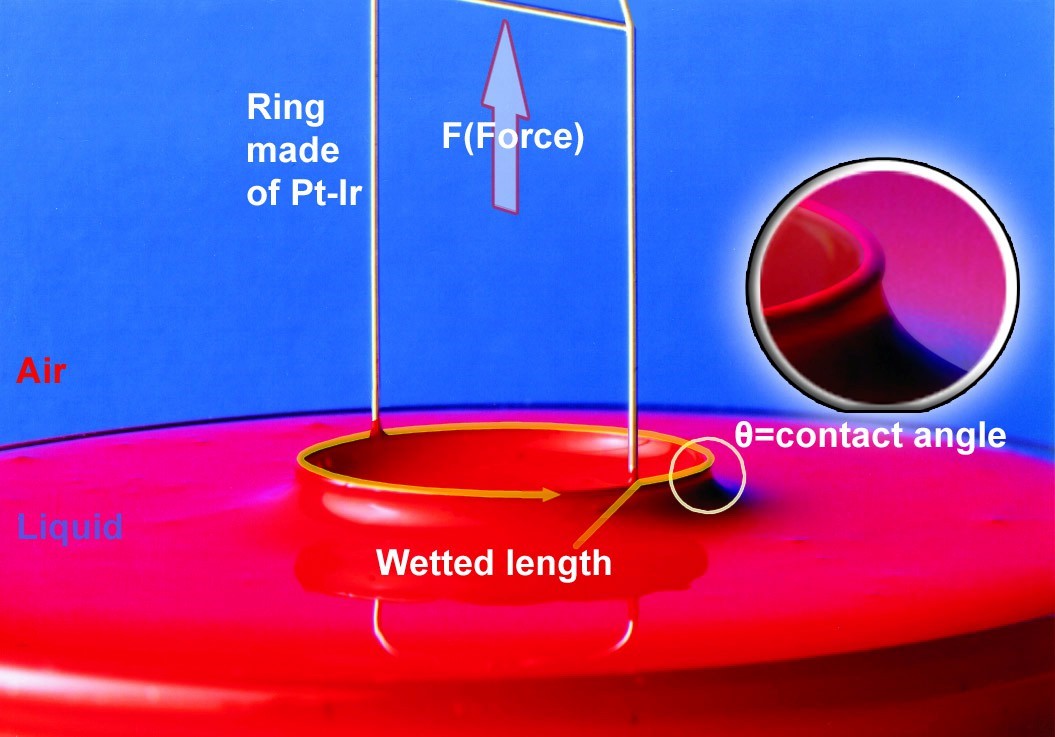
This question—heard countless times by application experts — is fundamental for anyone measuring surface or interfacial tension: Should I use the Du Noüy ring or the Wilhelmy plate?
While tradition, standardization, and familiarity have long favored the ring method, a deeper understanding of surface phenomena—especially in the presence of surfactants—reveals important limitations that shift the preference toward the plate method, particularly for equilibrium surface tension measurements.
Part 1: The Ring Method – Once Standard, Now Situational
Historically, the Du Noüy ring has been more widely used. Its prevalence in ASTM and other standards stems from the days when manual ring tensiometers dominated the field.
However, for modern applications—especially involving surfactants—the ring method introduces critical challenges that compromise accuracy.
Surface Perturbation – The Non-Equilibrium Problem
The ring method involves pulling the ring through the surface or stretching the surface during oscillation to find the maximum force. This inherently disturbs the surface, preventing it from reaching or maintaining equilibrium.
This doesn’t impact measurements of pure liquids, whose surface tension remains constant. But surfactant solutions are a different story:
-
- Surfactants need time to adsorb and orient at the interface.
- If the surface is expanding during measurement, these molecules cannot keep up.
- The result: the surface is not at equilibrium, and the measured tension is falsely high.
Example: Pull Rate vs. Measured Surface Tension
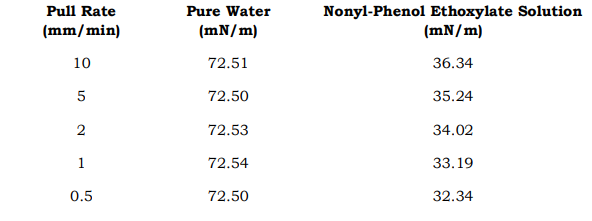
-
- Pure water’s surface tension remains consistent.
- The surfactant solution shows a clear dependency on pull rate—a hallmark of non-equilibrium conditions.
- True equilibrium surface tension (verified independently) is 30.12 mN/m.
Correction Factors Required
Another complication with the ring method is the need for mathematical corrections. The ring pulls up a meniscus, and part of the force recorded is due to capillary lift, not surface tension.
This requires correction factors like those from Harkins and Jordan, which consider:
-
- The shape of the meniscus
- Density of the liquid
- Ring dimensions
Failure to apply these corrections can lead to systematic overestimation— typically around 7% error.
Mechanical Precision – Ring Shape Matters
Rings must be perfectly round and parallel to the liquid surface. Even minor deformations or bends can:
-
- Skew the force distribution
- Prevent proper meniscus formation
- This leads to measurement failure or artificially high readings
Maintaining this level of precision—especially over time—is difficult, even with high-end instrumentation.
Part 2: The Plate Method – Preferred for Equilibrium Surface Tension
The Wilhelmy plate approach addresses nearly all of the ring method’s shortcomings for surface tension measurements:
- True Equilibrium Conditions
Unlike the ring, the plate:
-
- Is lowered gently into the surface and held in position
- Does not pull or stretch the surface during measurement
- Allows the surface to fully equilibrate with surfactants
This makes it ideal for measuring equilibrium surface tension, particularly for:
-
- Large-molecule surfactants
- Amphoteric surfactants
- Fluorosurfactants—all known for slow adsorption kinetics
Even a short waiting period (~1 minute) can ensure accurate, stable readings.
No Correction Factor Required
Because the plate:
-
- Forms a meniscus only at its edges
- Is not pulled through the liquid
- Can be positioned flush with the surface
…no correction for additional lifted liquid is needed. The measured force
directly corresponds to the surface tension using a known perimeter.
Easier Mechanical Handling
The plate is a flat platinum coupon, making it:
-
- Easier to keep clean
- Easier to align
- Easier to repair (platinum is ductile)
This reliability enhances repeatability and reduces measurement variability.
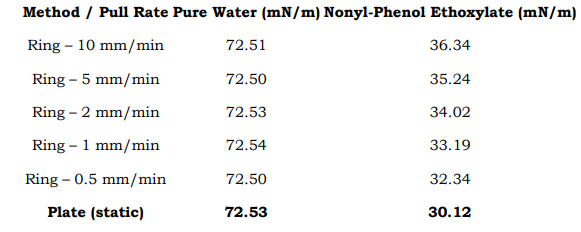
Side-by-Side Comparison with Plate Data Added
Part 3: Interfacial Tension – Where the Ring Still Rules
While the Wilhelmy plate is superior for surface tension, things change when measuring interfacial tension (IFT) between two immiscible liquids (e.g., oil and water).
Why the Ring is Better for IFT:
-
- A plate cannot easily form a zero contact angle at a liquid-liquid interface.
- Therefore, to use a plate, you must pull it through the interface— reintroducing the very issues the ring already handles better.
- The ring’s larger perimeter also gives it ~3× greater force signal compared to a plate, improving sensitivity and accuracy in IFT measurements.
That said, the non-equilibrium issue still applies: surfactants at the interface may not have equilibrated, so caution and equilibration time are still critical.
Final Thoughts – Don’t Measure the Wrong Thing by Accident
It is essential to match the method to the type of measurement needed:
-
- Use the ring for:
- Interfacial tension between liquids
- Compliance with certain legacy standards (e.g., ASTM D971)
- Use the plate for:
- Accurate surface tension of any solution
- Particularly for solutions with surfactants
- When true equilibrium values are needed
- Use the ring for:
Conclusions
- Rings are best reserved for interfacial tension and for complying with legacy standards.
- Plates are preferred for measuring equilibrium surface tension, especially in systems involving surfactants.
- Always consider whether you’re measuring at equilibrium—don’t let non-equilibrium measurements mislead you into thinking you have accurate surface tension data.

 Surface Tension Instruments
Surface Tension Instruments  Foam Analyzers
Foam Analyzers  High Pressure Reactors and Pilot Plants
High Pressure Reactors and Pilot Plants  Material Testing Service
Material Testing Service  Emulsion & Suspension Analysis
Emulsion & Suspension Analysis